Draw Cis And Trans Isomers Of 2 Butene
 | Shapes, Reactions & Biopolymers Examples of Multiple Choice Questions |
- 1.
- Two isomeric forms of a saturated hydrocarbon
- (a) have the same structure.
- (b) have different compositions of elements.
- (c) have the same molecular formula.
- (d) have a different content of the isotopes of hydrogen.
- (e) react vigorously with one another.
- 2.
- Which of the following hydrocarbons does not have isomers?
- (a) C7H16
- (b) C6H14
- (c) C5H10
- (d) C4H8
- (e) C3H8
- 3.
- The name of the alkane isomer of cis-3-hexene is:
- (a) 2-methylpentane
- (b) 3-methylpentane
- (c) n-hexane
- (d) 2,3-dimethylbutane
- (e) cyclohexane
- 4.
- How many aromatic isomers of dibromobenzene exist?
- (a) 2
- (b) 3
- (c) 4
- (d) 6
- (e) 8
- 5.
- Which one of the following compounds is an isomer of CH3CH2CH2CH2OH?
- (a) CH3CH2CH2OH
- (b) CH3CH(OH)CH3
- (c) CH3CH2CH2CHO (Note: This is one way to write an aldehyde.)
- (d) CH3CH2CH2CH3
- (e) none of the above
- 6.
- Which of the following compounds is a functional group isomer of C2H5OH, ethanol (ethyl alcohol)?
- (a) ethanal, CH3CHO
- (b) acetic acid, CH3COOH
- (c) diethyl ether, (C2H5)2O
- (d) dimethyl ether, (CH3)2O
- (e) propanol, C3H7OH
- 7.
- For which of the compounds below are cis-trans isomers possible?
CH3CH=CH2 CH3CH=CHCH2CH3 CH3CH=CHCH3 (1) (2) (3) - (a) only 2
- (b) both 1 and 2
- (c) both 2 and 3
- (d) all three
- (e) only 3
- 8.
- Which of the following does NOT exhibit geometric isomerism? (Hint: draw them!)
- (a) 4-octene
- (b) 2-pentene
- (c) 3-hexene
- (d) 2-hexene
- (e) 1-hexene
- 9.
- Which of the following compounds displays optical isomerism?
- (a) CH2(OH)-CH2(OH)
- (b) CH3-CHCl-COOH
- (c) CH2=CHCl
- (d) CHCl=CHCl
- (e) CH3-O-C2H5
- 10.
- How many isomeric alkanes of the molecular formula C5H12 are there?
- (a) 1
- (b) 2
- (c) 3
- (d) 4
- (e) 5
- 11.
- How many alcohols are structural isomers with the formula: C5H11OH?
- (a) 5
- (b) 6
- (c) 7
- (d) 8
- (e) 9
- 12.
- What is the relationship between the structures shown?
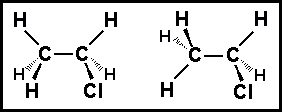
- (a) structural isomers
- (b) geometric isomers
- (c) conformational structures
- (d) identical structures
- (e) optical isomers
- 13.
- Which of the following statements concerning conformations is (are) TRUE?
- (1) Ethane has an infinite number of conformations.
- (2) The eclipsed conformation of a molecule is slightly more stable and energetically favored than the staggered conformation.
- (3) A conformation is one specific geometry of a molecule.
- (a) 1 only
- (b) 2 only
- (c) 1 and 2
- (d) 2 and 3
- (e) 1 and 3
- 14.
- Which of the following statements is FALSE regarding the reaction between Cl2 and C2H6?
- (a) It is a substitution reaction.
- (b) The reaction will give a single product of C2H5Cl.
- (c) The reaction mechanism involves free radicals.
- (d) The reaction can be initiated with either sunlight or heat.
- (e) The first step in the mechanism is the cleavage of the Cl-Cl bond to give chlorine atoms.
- 15.
- Which of the following will undergo an addition reaction with chlorine?
- (a) CH3CH2CH2CH3
- (b) CH3CH2CH=CHCH3
- (c) C6H6
- (d) CH3CH2COOH
- (e) CH3CH2OH
- 16.
- What is the expected product formed from the reaction between 2-butene and Cl2?
- (a) 1-chlorobutane
- (b) 2-chlorobutane
- (c) 2,3-dichlorobutane
- (d) 2,2-dichlorobutane
- (e) 3,3-dichlorobutane
- 17.
- The reaction of ethyne with which of the following gives CH2Br-CHBrCl?
- (a) HCl, then HBr
- (b) HCl, then Br2
- (c) Cl2, then HBr
- (d) Cl2, then Br2
- (e) H2, then Br2
- 18.
- Dehydration of an alcohol leads to the formation of an _____ .
- (a) alkene
- (b) alkane
- (c) alkyne
- (d) alkyl halide
- (e) aldehyde
- 19.
- A reaction in which a carboxylic acid reacts with a base to form a salt and water is called _____ .
- (a) ionization
- (b) esterification
- (c) hydrolysis
- (d) saponification
- (e) neutralization
- 20.
- How many moles of sodium hydroxide will react with one mole of:
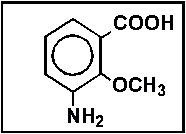
- (a) 5
- (b) 4
- (c) 3
- (d) 2
- (e) 1
- 21.
- Ethanol can be oxidized stepwise. What is the first stable intermediate product when ethanol is oxidized with a mild oxidation agent?
- (a) CH3COOH
- (b) CO2
- (c) CH3CHO
- (d) CH3CH2OH
- (e) CH3OCH3
- 22.
- Which of the following alcohols forms a ketone when oxidized?
- (a) 1-propanol
- (b) methanol
- (c) 2-methyl-2-propanol
- (d) 2-propanol
- (e) all of the above
- 23.
- What is the sum of the coefficients in the balanced equation for the complete combustion of 2-methylbutane? Use smallest whole number coefficients. Do not forget coefficients of 1.
- (a) 10
- (b) 13
- (c) 17
- (d) 20
- (e) 23
- 24.
- The organic starting materials for the preparation of an ester could be _______ .
- (a) an acid and an alcohol
- (b) a ketone and an alcohol
- (c) an alkane and a ketone
- (d) only an acid
- (e) an amine and an acid
- 25.
- Hydrolysis (saponification) of a fat would yield ______ .
- (a) water and an alkene
- (b) ethanol and propanoic acid
- (c) glycerol and soap
- (d) ethanol and a soap
- (e) a triester of glycerol with fatty acids
- 26.
- The segment -CH2CH2CH2CH2CH2CH2- represents the polymer named _______ .
- (a) polybutylene
- (b) polyhexene
- (c) polypropylene
- (d) polystyrene
- (e) polyethylene
Answers:
1. (c) 2. (e) 3. (e) 4. (b) 5. (e) 6. (d) 7. (c) 8. (e) 9. (b) 10. (c) 11. (d) 12. (c) 13. (e) 14. (b) 15. (b) 16. (c) 17. (b) 18. (a) 19. (e) 20. (e) 21. (c) 22. (d) 23. (d) 24. (a) 25. (c) 26. (e)

 Click here to return to the top.
Click here to return to the top.
 Choose your next chapter:
Choose your next chapter: | Atomic Structure | Chemical Periodicity | Chemical Bonding | Molecular Structure/Covalent Bonding Theories| Molecular Orbital Theory |
| Acids/Bases/Salts - Theory & Rxns | Acids/Bases/Salts - Calculations (including balancing redox rxns) | Gases | Solids & Liquids | Solutions |
| Aqueous Equilibrium - Slightly Soluble Salts | Electrochemistry | Metallurgy | Metal Properties & Rxns | Nonmetals & Metalloids |
| Coordination Compounds | Nuclear Chemistry | Organic Chem - Formulas/Names/Properties | Organic Chem - Shapes/Rxns/Biopolymers |

 To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
To report any corrections, please e-mail Dr. Wendy Keeney-Kennicutt.
Draw Cis And Trans Isomers Of 2 Butene
Source: https://www.chem.tamu.edu/class/fyp/mcquest/ch28.html
Posted by: williamsalannow.blogspot.com

0 Response to "Draw Cis And Trans Isomers Of 2 Butene"
Post a Comment